We help brand owners design, manufacture, and test environmentally-friendly glass packaging without losing brand recognition and luxury appeal.
We work with companies throughout the glass supply chain on environmental projects such as recycling schemes, glass re-use and specialist raw material recovery, lightweighting of glass products, and environmental impacts.
We regularly batch test pharmaceutical glassware for a range of global manufacturers, importers and suppliers to verify compliance to industry standards, dimensional specifications and performance properties.
We provide this analysis for pharmaceutical companies to determine the amount of elemental migration and elemental impurities in drug products. The test method involves an accelerated test procedure to encourage leaching of specified elements from the glass inner surface. These elements are defined by the ICH Q3D as elements to be quantified with defined limits within the document itself.
We offer a unique perspective of glass as a material within the context of recycling, sustainability and the circular economy. Our experts provide advice about environmental and sustainability issues, and undertake large consultancy projects involving multiple partners, such as universities, funding agencies and companies. The work we undertake in this area includes audits of new and existing processes, reviews of the availability and demand of materials, and studies of process and energy efficiencies.
Our glass materials, production and processing experts support the development of important industrial processes across various glass industries and sectors. We are the leading authority on glass products, glass raw materials and glass contact materials, with unrivalled knowledge and facilities.
Our team are experts in the chemical and physical properties of glass materials. We work with manufacturers to optimise production, troubleshoot issues and support the development of novel glasses. We offer analysis of glass colour, dilatometry and thermal expansion, electrical properties, density, light transmission and absorbance, littleton softening point, refractive index, refractory corrosion and stability, temperature-viscosity, and thermal and solar properties.
Our extensive fit-for-purpose tests determine whether your glass products are suitable against international, national, industry or in-house standards and specifications. They can also prevent customer complaints and reduce waste.
We can ensure the quality standards and performance of your glass product through a range of glass quality testing methods and technical support – helping you maintain a positive brand image and prevent product recalls.
We help glass manufacturers measure and verify the critical dimensions of a product against specific requirements. Our intricate processes identify any dim issues related to packaging and decorating glass containers, such as applying lids and labels.
Foreign bodies, objects, glass fragments, glass particles and other contaminants found in products are a serious issue at any point in the food and drink, pharmaceutical and cosmetics supply chains. Through analysis, we can identify whether the foreign glass object was introduced during the manufacturing, processing or filling stage, or potentially by the customer after purchase. And we can work with you to resolve the issue to potentially prevent it from happening again.
Delamination of glass occurs when visible flakes or glass lamellae appear in glass. It’s a common cause of product recall, at a great cost to manufacturers.
We are world-leading experts in developing and manufacturing controlled dissolution glasses, designed to deliver specific chemistries to a limited location over a controlled timeframe. Dissolution behaviour is an important characteristic of glasses used in biomedical applications. However, these glasses also have other uses, such as water treatment applications and consolidating or sealing loose rocks and soil.
Using our characterisation tools and glass materials expertise, we can determine a wide range of properties relevant to your materials – ensuring your product specifications are met and your materials are fit for purpose. Products we perform materials characterisation on include consumer glassware, flat glass, container glass, optical and photonic materials, biomedical materials and refractory materials.
We can help you develop laser processing techniques tailored specifically to glass materials. We have specialist expertise in areas including laser welding technologies, glass laser processing and cutting, additive manufacture (3D printing) and targeted property modification. Working together, we can develop new products for sectors such as micro-machining, biomedical technologies and advanced optics. We support and collaborate with partners and customers spanning academia and industry.
Our glass production and processing experts provide manufacturers, suppliers, brand owners and customers with line and process audits to optimise glass production, processing, and food and drink operations. We work with companies involved in all stages of glass supply, from raw materials through to the end product. In emergencies, we can undertake site visits at short notice to identify issues quickly, allowing production to resume and minimising financial losses.
Glass failure (or fracture) analysis is a powerful tool combining glass fractography and microscopy to diagnose and determine the root cause of a breakage or product failure. We also complete preventative quality assessments to potentially stop issues occurring in the future. We provide analysis for products spanning all sectors, including food and drink packaging, domestic glass, furniture, scientific glassware and pharmaceutical packaging.
Our experts can verify that your glass complies with international and industry standards. Our durability analysis and testing ensures it’s fit for purpose, it provides the appropriate level of chemical resistance, and it meets all required standards. This is vital for chemically sensitive products such as parenteral pharmaceuticals. However, it is also important to ensure the safety of packaging, preparatory surfaces and vessels for foods and drinks.
Our technical experts analyse glass composition and causes of defective glass to quickly resolve your production issues. We undertake composition analysis of glass inclusion defects, stone, cord, knot, bubbles and blisters, and we examine surface contamination, delamination, misting and blooms. We also assess forming defects in glass manufacturing and quality concerns in finished products.
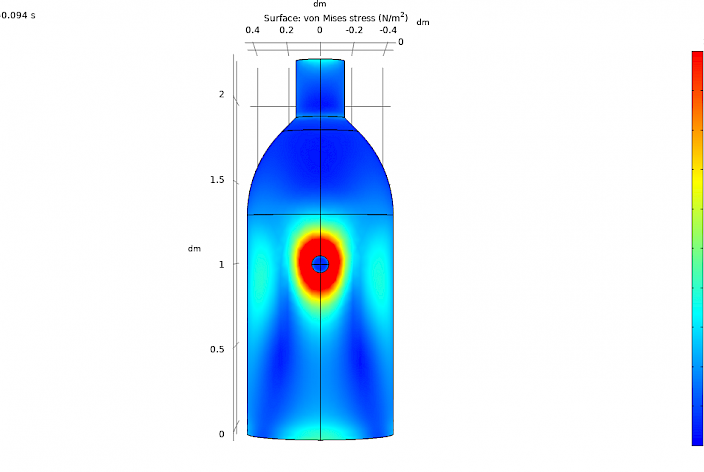
We use finite element analysis (FEA) modelling to provide insights into a vast range of processes and scenarios. We combine our FEA software capability with expertise in glass materials and glass manufacture to model heat transfer and stress generating processes, ranging from furnaces to laser processing. Our finite element analysis modelling provides vital information that allows you to minimise costs and reduce the probability of failures.
We provide ISO/IEC 17025-accredited composition analysis, enabling glass manufacturers to confirm that glass has been made as intended, and potentially determine the cause of a problem. Our methods are created specifically for glass, so they’re accurate and reproducible.
Our experts provide a series of small-scale melting trials to confirm the suitability of new raw materials in glass production. We use comparative melting trials to establish differences between compositions or refining systems. The normal trial we offer is based on melting four glasses. The melts are all made to produce 250g of glass. We study the melting and refining of each formulation for 30, 60, 90 and 120 minutes, at a temperature comparable with the customer’s melting temperature.